Projects
We’ve considered well over 1,000 ideas for new medical devices since our establishment. We’ve completed over 210 collaborative workshops and 120 R&D projects.
Our clients come from a range of professions: surgeons wanting to improve the lives of patients; researchers needing assistance to get their research out of the labs and into the hands of end-users; industry from startups to established businesses who are diversifying their product line; and the everyday inventor who sees a problem and has an idea to solve it.
Projects have included special glasses that help frequent flyers and shift workers adjust their sleep cycle, a cancer-detecting probe that is improving surgical outcomes and a device to assist orthopedic surgeons when fixing bone fractures.
DeflectaCath tipped to redefine urological care.
An Australian clinical surgeon has collaborated with the Medical Device Partnering Program (MDPP) at Flinders University to develop a novel catheter for safely navigating the intricate curves and contours of the male urinary tract.
Urological surgeon Associate Professor Ian Middleton developed "DeflectaCath," a silicone catheter equipped with a unique deflection mechanism in its tip, to simplify the insertion procedure for clinicians and improve the health outcomes for patients.
DeflectaCath provides a safe and comfortable catheterisation solution for men managing health conditions or undergoing medical procedures. It allows practitioners to manoeuvre the catheter through the length and curvature of the urethra, effectively minimising the risk of potential complications.
A/Prof Middleton said it was exciting working with the MDPP team to prototype a standout medical device that improves the catheterisation experience for both healthcare professionals and their patients.
“Working closely with the MDPP team has allowed us to concentrate on innovating the design of DeflectaCath, ensuring an easy and intuitive insertion and placement for medical staff while reducing the associated health risks of urethral trauma for patients,” said A/Prof Middleton.
The unique deflection mechanism in DeflectaCath's tip enhances device maneuverability, minimising the chances of urethral damage or the formation of false passages that can occur from urethral wall perforation. This advancement is especially critical with studies reporting that catheterisation poses challenges in around one in five cases.
“We’re extremely excited to have developed an efficient and effective catheterisation alternative which is unlike any of the current catheters being used by clinicians,” said A/Prof Middleton. “Thanks to the MDPP team, DeflectaCath now includes manual control, allowing medical practitioners to navigate a patient's anatomy throughout the entire procedure. This capability represents a game-changing advancement for both practitioners and patients,”
Professor Karen Reynolds, director of MDPP, highlighted that DeflectaCath's potential transformative impact on healthcare delivery marks a new era of enhanced patient outcomes and improved quality of care in urology.
“Our team takes pride in supporting innovators like Ian to find novel solutions and DeflectaCath represents a significant breakthrough in urological procedures,” said Prof Reynolds. “Helping to revolutionise the catheterisation process reflects our steadfast commitment to innovation and advancing patient care and outcomes.”
This project involved resources from the Medical Device Research Institute (MDRI) at Flinders University, the Australian National Fabrication Facility (ANFF) and the team at MDPP provided 250 hours towards proof-of-concept research, prototyping and product validation. Fostering collaborations between researchers, industry, end-users, and government to develop medical technologies with global market potential, the MDPP is an ideas incubator. Do you have an innovative medtech idea? Submit your idea through our website and a member of the team will get in touch. For any other questions please contact us on 08 8201 5029 or email This email address is being protected from spambots. You need JavaScript enabled to view it..

Photo: A transparent model of the urethral anatomy has been produced to demonstrate how the deflection mechanism can allow the catheter to bypass a false passage and prevent new trauma to the urethra.
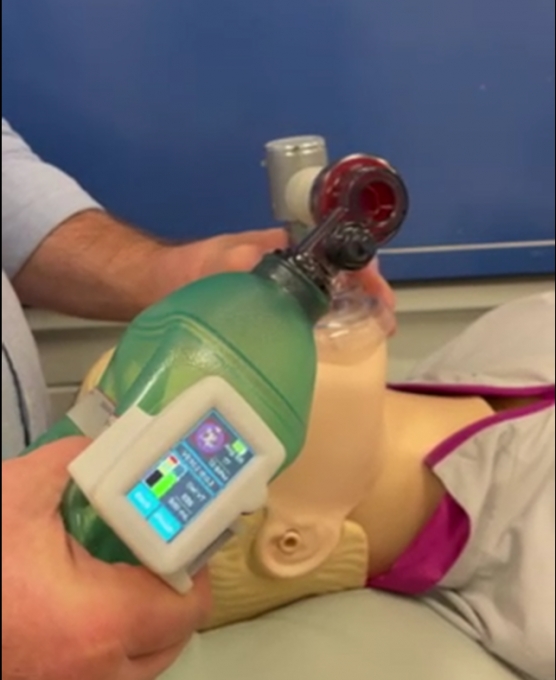
Ventilation management during life-saving resuscitation is being transformed by a local start-up, Abtulus®, who have partnered with the Medical Device Partnering Program (MDPP) at Flinders University to develop a novel Bag Valve Mask (BVM) guidance system.
Engineered by Dr Siavash Noor (Ahmadi Noorbakhsh) of Abtulus®, VentiWatch is a practical, simple and reusable ventilation management solution supporting medical professionals and first responders with the delivery of precise and efficient ventilation during resuscitation.
Revolutionising the landscape of healthcare delivery, innovator Hayley Saddington has joined forces with Flinders University's Medical Device Partnering Program (MDPP) to build a cost-effective, clinically guided digital software application tailored for supporting in-home rehabilitation.
The co-founder of Peak Medical, Hayley is a visionary in her field who has exploited artificial intelligence to create an innovative technology which empowers in-home patients to progress their rehabilitation with the confidence of precise clinical guidance.
create an innovative technology which empowers in-home patients to progress their rehabilitation with the confidence of precise clinical guidance.
Replicating the physiotherapy experience, the technology employs an evidence-based clinical approach, directing individuals to customised exercises for continuous improvement between appointments.
Darius Chapman is founder and Managing Director of Cardiovascular Technology Services Pty Ltd, a bootstrapped medical device company selling products to major multinational companies and customers in an international market from Blackwood, South Australia. With a small and agile team of 2, CTS identifies small but important problems to help patients with cardiac electrical abnormalities.
Contact Darius at Darius Chapman This email address is being protected from spambots. You need JavaScript enabled to view it.
Who are you?
Our company has been working on the development of a smart mechanical device to assist with rehabilitation following shoulder surgery. The device was created to allow passive shoulder ROM in the home or clinical setting, by driving the arm along a surface to allow safe mobilisation in the early stages of rehabilitation.
Designed by a Dental Hygienist to be the ultimate mouldable mouthguard. NeoMorph is a hybrid, combining the best qualities of the professionally made mouthguard with the convenience of a “boil & bite”. No more bulky, ill-fitting guards that dislodge on impact or bond to orthodontic appliances. NeoMorph is a pioneering over-the-counter sports mouthguard delivering a level of protection and custom fit previously unexpected in a reformable mouth guard.
Approximately 76,000 pregnant women and 500,000 babies die from pre-eclampsia across the world every year. Women in developing countries are seven times more likely to suffer from the condition due to delayed diagnosis and a lack of access to hospitals.
Causes of pre-eclampsia are not entirely understood, but if left untreated it can lead to blood clots, organ failure, and death. Rapid, affordable, and accurate diagnostic tools are therefore essential in aiding treatment.
Now, with the help of the Medical Device Partnering Program, a portable diagnostic device being developed by Professor Benjamin Thierry, Dr Duy Tran and a team from the University of South Australia’s Future Industries Institute (UniSA FII) has shown it could pose a solution.
What started as a family injury has turned into a rehabilitation game-changer receiving global attention.
The Prohab Connected Healthcare Device, the brainchild of physiotherapist Lyndon Huf, is an innovative rehabilitation device that accurately measures the force-generating capability of an individual’s muscle, in order to guide and personalise prescription of exercise and rehabilitation programs .
When Scott Blackburn injured himself badly in an accident, breaking both arms and his leg, he was surprised when the Ambulance arrived and treated him with a piece of cardboard.
Thinking there must be a better way, Scott established Fluoro Medical and created their first product, the CAS Splint. He then turned to the Medical Device Partnering Program (MDPP) for assistance to develop their new innovation.
The CAS Splint is an innovative, foldable splint for fractured limbs that requires no training to use and does not have to be removed during x-rays.
The MDPP undertook an end-user trial and survey to validate the current design of the splint and made recommendations for design modifications. The MDPP also provided a market intelligence report to Fluoro Medical.
When ResMed, a world-leading connected health company, engaged with the Medical Device Partnering Program in 2010, they wanted to investigate the feasibility of measuring specific signals from the head or face of a patient with Obstructive Sleep Apnoea (OSA).
ResMed sought to develop a non-intrusive device to monitor the cardiac health of OSA patients which would enable the monitoring of health so treatment could be administered with greater insight.
Obstructive Sleep Apnoea (OSA) is a condition where a person's upper airway becomes blocked during sleep and impacts more than 936 million people worldwide. Not only does OSA impact sleep quality, but patients are at a greater risk of developing cardiovascular disease and other heart problems.
The MDPP project resulted in a collaboration between ResMed and Flinders University, with a PhD scholarship funded to further research and development in this area.
When Orthopaedic Surgeon, Dr Matthew Liptak started noticing inconsistent outcomes in the rehabilitation of his patients after total knee replacement, he decided to take things into his own hands.
He identified a gap in the market for a simple and effective device to encourage, motivate and monitor the rehabilitation of his patients post-surgery.
Dr Liptak first approached the MDPP in 2013 for assistance with the development of Maxm Skate, a skate-like device which straps to a patient’s foot. Whilst he had an initial prototype of the skate, he needed R&D assistance to develop a sensor which would record the user’s range of movement.
Detecting cancer is set to become safer and more accurate with the development of a new cancer-detecting probe.
The probe, an invention of University of South Australia researchers Professor Benjamin Thierry and Dr Aidan Cousins, provides a non-radioactive alternative to map how cancer has disseminated through the body.
Following initial bench-top testing of the device, the team first partnered with the MDPP in 2013, seeking out electronic engineering expertise and advice from end users and commercial experts.
Page 1 of 2