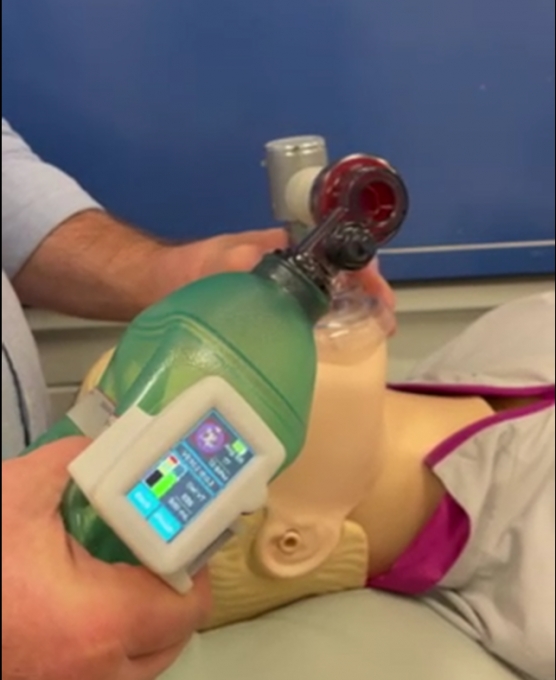
Ventilation management during life-saving resuscitation is being transformed by a local start-up, Abtulus®, who have partnered with the Medical Device Partnering Program (MDPP) at Flinders University to develop a novel Bag Valve Mask (BVM) guidance system.
Engineered by Dr Siavash Noor (Ahmadi Noorbakhsh) of Abtulus®, VentiWatch is a practical, simple and reusable ventilation management solution supporting medical professionals and first responders with the delivery of precise and efficient ventilation during resuscitation.
Read more

DeflectaCath tipped to redefine urological care.
An Australian clinical surgeon has collaborated with the Medical Device Partnering Program (MDPP) at Flinders University to develop a novel catheter for safely navigating the intricate curves and contours of the male urinary tract.
Urological surgeon Associate Professor Ian Middleton developed "DeflectaCath," a silicone catheter equipped with a unique deflection mechanism in its tip, to simplify the insertion procedure for clinicians and improve the health outcomes for patients.
Read more

Automatic Smart Pressure Cushion
For people with reduced mobility who spend extended periods seated, pressure injuries aren’t just uncomfortable - they can be painful, life‑altering, and even life‑threatening.
Adelaide innovator Mossa Medical is tackling this challenge through the ARIIA x MDPP Ideas Incubator, coordinated by Aged Care Research & Industry Innovation Australia (ARIIA), with technical support from Flinders University’s Medical Device Partnering Program (MDPP).
Read more

Reminu
A revolutionary digital platform is set to transform aged care by enabling organisations to deliver truly client-centred care - improving resident wellbeing and providing peace of mind for families across Australia.
Founded by New Word Order, Reminu was developed through a strong partnership with Aged Care Research & Industry Innovation Australia (ARIIA) and Flinders University’s Medical Device Partnering Program (MDPP) via the ARIIA x MDPP Ideas Incubator.
The platform provides user-friendly software accessible on handheld devices and desktops to document life stories, preferences, and future care plans. It gathers information directly from individuals and trusted sources, ensuring wishes are shared with care...
Read more

A cutting-edge collaboration between North Eastern Community Hospital and the ARIIA x MDPP Ideas Incubator has resulted in several novel prototyped food cutters, enabling kitchen staff to deliver precise IDDSI Soft and Bite-Sized portions (1.5 x 1.5 cm) for residents facing swallowing and chewing challenges.
Through 250 hours of technical expertise from the Medical Device Partnering Program (MDPP) and 30 hours of commercial research via Aged Care Research and Industry Innovation Australia (ARIIA), the project produced three prototypes for North Eastern Community staff to begin trials that can potentially deliver safer, faster, and more consistent food preparation. The prototype cutting tools...
Read more

Jack Menzies, founder of Freeline Pty Ltd and an experienced occupational therapist, has developed a lifechanging innovation—the Mobile Step-Climbing Shower Commode—which has the potential to assist older Australians safely overcome bathroom steps and thresholds.
The solution was created through a close collaboration with Aged Care Research and Industry Innovation Australia (ARIIA) and Flinders University’s Medical Device Partnering Program (MDPP) as part of the now concluded ARIIA x MDPP Ideas Incubator.
Read more

An Australian medtech startup is harnessing frontline paramedic experience to develop Pulse Tile, a promising device set to accelerate cardiac arrest responses.
Founder and CEO, Elleesha King, a seasoned paramedic who's attended countless cardiac arrests, repeatedly saw the same issue: manual pulse checks are slow, stressful and subjective, causing critical delays in starting CPR or defibrillation. Pulse Tile has the potential to fill that gap – a compact device that quickly confirms the presence or absence of a pulse, empowering responders to act decisively.
Read more

Revolutionising the landscape of healthcare delivery, innovator Hayley Saddington has joined forces with Flinders University's Medical Device Partnering Program (MDPP) to build a cost-effective, clinically guided digital software application tailored for supporting in-home rehabilitation.
The co-founder of Peak Medical, Hayley is a visionary in her field who has exploited artificial intelligence to create an innovative technology which empowers in-home patients to progress their rehabilitation with the confidence of precise clinical guidance.
Replicating the physiotherapy experience, the technology employs an evidence-based clinical approach, directing individuals to customised exercises for continuous improvement between appointments.
Read more

Approximately 76,000 pregnant women and 500,000 babies die from pre-eclampsia across the world every year. Women in developing countries are seven times more likely to suffer from the condition due to delayed diagnosis and a lack of access to hospitals.
Causes of pre-eclampsia are not entirely understood, but if left untreated it can lead to blood clots, organ failure, and death. Rapid, affordable, and accurate diagnostic tools are therefore essential in aiding treatment.
Now, with the help of the Medical Device Partnering Program, a portable diagnostic device being developed by Professor Benjamin Thierry, Dr Duy Tran and a team from the University...
Read more




















